With more than a billion students withdrawn from school and children without critical stages of social development, children have assumed a massive burden amid the pandemic. Now kids seem to be taking one more: testing a vaccine before it is shown to be safe in adults.
This week, Chinese vaccine maker Sinovac announced that it will launch a phase I and II clinical trial later this month to test its vaccine in more than 500 children ages 3 to 17 in China. The trial will test the safety of the vaccine and will also examine whether it elicits an immune response among participants.
Experts say the need to conduct COVID-19 vaccine safety trials specifically in children is clear, but that conducting trials before a vaccine has been shown to be safe and effective among adults represents unexplored and ethically tense territory. .
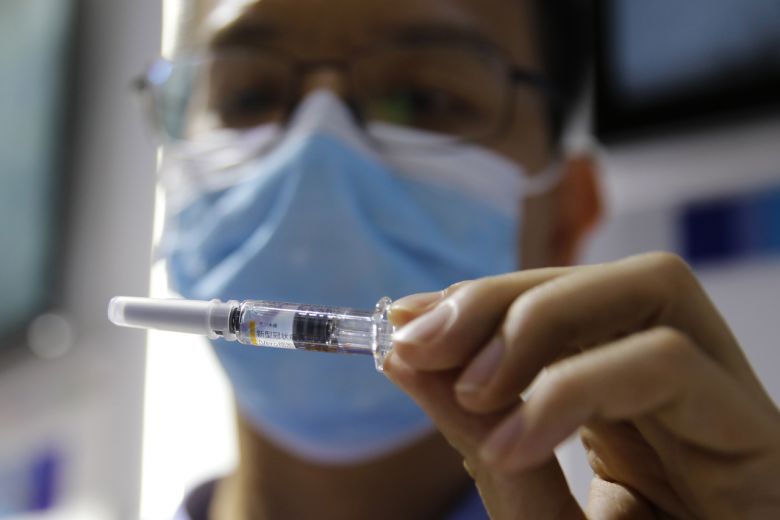
CoronaVac
The Synovac candidate vaccine, called Conovac, is based on the most proven approach to vaccine development, using an inactive version of COVID-19 to teach the immune system to develop disease tolerance.
After beginning vaccine development on January 28, the company is now among the leading group of companies in the global vaccine race and is one of only nine vaccine manufacturers globally to achieve phase III trials.
But Sinovac has also shown its willingness to avoid some normal safety procedures when deploying its vaccine.
The company is one of several Chinese vaccine manufacturers involved in the country’s controversial emergency use program, which has exposed hundreds of Chinese citizens, including front-line medical staff and other select population groups, despite the Phase III clinical trials, which are designed to show that a vaccine is effective and safe, have been completed. In early September, Dr. Yin Weidong, Sinovac’s chief executive, told Reuters that the company had already injected up to 3,000 Sinovac employees and their families who are not part of the clinical trials.
He explained that retrieving Phase III results would normally be a vaccine manufacturer’s “first port of call” before considering trials in children, but that Sinovac’s decision was one of many examples where drug manufacturers They seem to have scrapped the usual playbook for developing a COVID. 19 Vaccine at a record rate.
Children and vaccines
Sinovac published the results of the phase I and II trials (studies designed to show that a drug is safe) on August 10 in a non-peer-reviewed article on the online health research repository MedRxIV. Sinovac also reported that its phase I and II trials were shown to be safe specifically among elderly volunteers in China in preliminary results on September 9.
These data alone would generally not be adequate to start trials in children, the experts said. Moore said it was possible that Chinese regulators had additional safety information from Sinovac that has yet to be released.
Sinovac did not respond to Fortune’s request for comment on this story.
Chinese skepticism
Sinovac had spoken about his desire to conduct vaccine safety trials in children since April, says Yanzhong Huang, a vaccine expert and senior global health researcher at the Council on Foreign Relations.
By starting these trials so early, Sinovac may aim to increase national confidence in a vaccine developed in China among a skeptical public, he says.
In the 2010s, a series of internal scandals in China’s vaccine industry undermined public confidence in the country’s efforts to produce a vaccine.
Sinovac wasn’t directly related to any of China’s substandard vaccines, but the company has been prone to missteps. In 2017, a Beijing court ruled that China’s pharmaceutical regulator received improper payments from vaccine manufacturers, including Sinovac. This prompted US regulators to freeze Sinovac’s share trading on Nasdaq, and Sinovac’s case is still pending in US courts.
Huang says China’s accelerated COVID-19 vaccine development process raises concerns about the safety and efficacy of vaccines made in China and the reliability of information released by the government. Such pressure may help explain why Sinovac is motivated to prove its vaccine is safe in children as soon as possible.