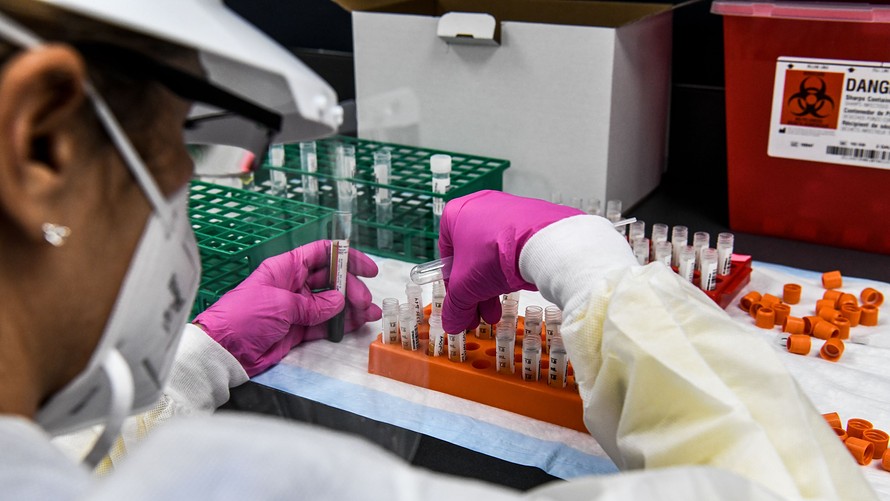
A coronavirus vaccine will not be a silver bullet against the COVID pandemic, but public health experts say it is an important step that could start the process of returning to normalcy. And four companies (along with their partners) are competing for first market entry with advanced stage testing underway.
Companies ranging from pharmaceutical giants to emerging biotechnologies, in the United States and abroad, are all part of this race. But the regulatory process can be a labyrinth, and the fundamental question of the scientific precision of a vaccine that appears to be distributed to hundreds of millions of people is fundamental.
“The FDA will not authorize or approve a vaccine that we have no confidence in giving to our families,” said Food and Drug Administration (FDA) Commissioner Stephen Hahn during a hearing in the US Senate on Wednesday.
That should have an impact on the position of the various companies that develop these vaccines.
Pfizer/BioNTech
Pfizer has been among the most optimistic companies in the COVID vaccine fray.
According to Pfizer CEO Albert Bourla, they are confident they will have a good idea, at least, whether or not their treatment works this fall.
“I think by the end of October we have a very good chance to find out if the vaccine works or not,” Bourla said during a virtual roundtable with Fortune earlier this month.
This boils down to what FDA Commissioner Hahn said during his testimony Wednesday: the need for large-scale trials to establish safety and efficacy. But there’s a caveat here: Hahn has also said the agency may consider an emergency use authorization, which differs from full approval and has lower standards, depending on data submitted by drug manufacturers. The next informed guidance for such clearances could raise that standard. An emergency clearance can be obtained sometime this fall, even if a full approval is not obtained.
Currently, Pfizer may be the first to know, at a minimum, if its COVID vaccine works, according to Bloomberg.
Moderna
Moderna caused quite a stir earlier this summer with its COVID vaccine candidate, mRNA-1273. It would be the first drug approved by upstart biotechnology if it crosses the regulatory finish line.
The science of business is interesting. Simply put, “mRNA technology” literally redesigns the biology of cells to fight various diseases. Moderna has had its share of skeptics as none of its medications and the technology that makes them possible have paid off in real life to date.
Chief Executive Officer Stephane Bancel told CNBC last week that he hopes to have enough data to know whether or not the vaccine is effective by November.
Johnson & Johnson
The COVID vaccine being developed by Johnson & Johnson’s pharmaceutical arm, Janssen, just entered phase 3 trials on Wednesday, the company said.
The J&J vaccine is a single dose, rather than multiple doses like the Pfizer and Moderna candidates. And the trial announced Wednesday is massive in scope. It will have 60,000 participants in at least eight countries, including the United States, South Africa and Brazil.
Success in drug development often depends on the first-to-market advantage. But the simplicity of use, one injection instead of two, during a pandemic could help J&J get better.
AstraZeneca and Oxford
British pharmaceutical giant AstraZeneca has partnered with Oxford University education giant for its own coronavirus candidate vaccine, AZD1222. That process hit a roadblock recently with a hiatus in a clinical trial led by a study participant who developed a serious side effect.
AstraZeneca CEO Pascal Soriot remained optimistic after that turn of events.
The sunny outlook, for now, appears to have been prophetic. On 13 September, UK health authorities recommended that the study be resumed. However, his situation in the United States and in trials in several other countries remains unstable. On Wednesday, US Department of Health and Human Services (HHS) Secretary Alex Azar said it was still on hold.